Accessibility Options:
What is Evidence Based Medicine?
Evidence-Based Medicine (EBM) is the conscientious, explicit, judicious and reasonable use of modern, best evidence in making decisions about the care of individual patients. EBM integrates clinical experience and patient values with the best available research information. (Sackett, 1997).
Evidence-Based Public Health is defined as the "conscientious, explicit, and judicious use of the current best evidence in making decisions about the care of communities and populations in the domain of health protection, disease prevention, health maintenance and improvement (health promotion)." In other words, "it is the process of systematically finding, appraising, and using contemporaneous research findings as the basis for decisions in public health." (Jenicek, 1997)
Subject Specialist
-
-

Saily Marrero
Nursing & Health Studies, Biology, and Psychology Librarian
305-284-2040
Please provide a brief description of the topics or issues you would like to discuss during our consultation.
The Steps in the EBP Process

Step 1: Assess – Start with the patient. A clinical problem or question arises from patient care.
Step 2: Ask – Create a relevant and answerable question.
Step 3: Acquire – Select the appropriat databases and conduct a search.
Step 4: Appraise – Evaluate the evidence for validity, risk of bias and applicability.
Step 5: Apply – Go back to the patient and incorporate the new information you found.
Related Guides
- Nursing & Health Studies Guide
- Evidence Based Practice Research Guide [Duke University Research Guide]
- EBM: Types of Studies [GWU Research Guide]
Further Reading
Jenicek M. (1997). Epidemiology, evidenced-based medicine, and evidence-based public health. Journal of epidemiology, 7(4), 187–197. https://doi.org/10.2188/jea.7.187
Sackett D. L. (1997). Evidence-based medicine. Seminars in perinatology, 21(1), 3–5. https://doi.org/10.1016/s0146-0005(97)80013-4
Sackett, D. L., Rosenberg, W. M. C., Gray, J. A. M., Haynes, R. B., & Richardson, W. S. (1996). Evidence Based Medicine: What It Is And What It Isn’t: It’s About Integrating Individual Clinical Expertise And The Best External Evidence. BMJ (Online), 312(7023), 71–72. https://doi.org/10.1136/bmj.312.7023.71
Young, J. M., & Solomon, M. J. (2009). How to critically appraise an article. Nature Clinical Practice. Gastroenterology & Hepatology, 6(2), 82–91. https://doi.org/10.1038/ncpgasthep1331
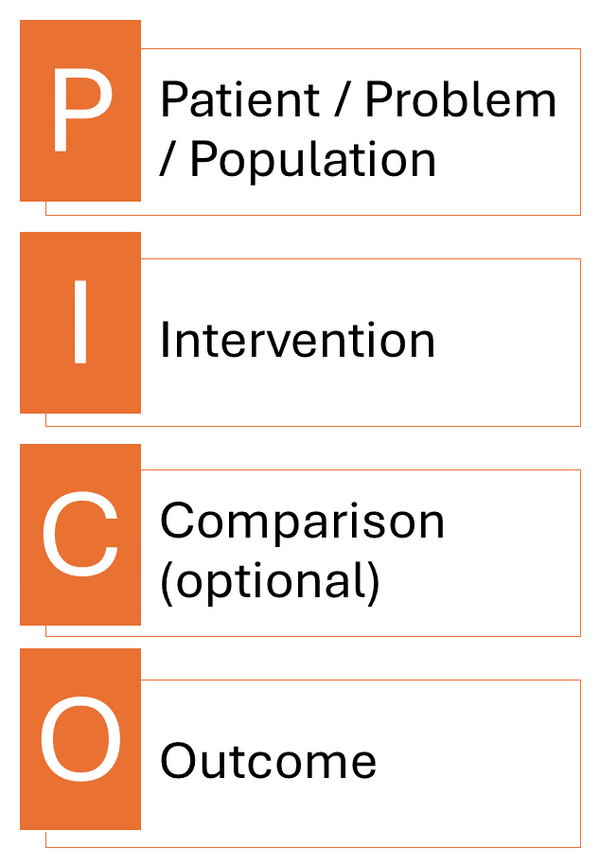
PICO Question
PICO is a framework used by clinicians when formulating a question. Each letter stands for and important part of the question: Patient, Intervention, Comparison, and Outcome.
Patient / Population / Problem: How would you describe the population or problem? What are the most important characteristics and factors? Is it important to note the biological sex and age of the patient?
Intervention / Exposure / Prognostic Factor: What intervention, exposure, or prognostic factor are you investigating? What do you want to do with this patient?
Comparison: What is the alternative you are considering? This could be comparing two different therapies, two dianostic tests, or comparing a drug to a placebo.
Outcome: What are you trying to accomplish, measure, improve, or affect?
PICO
Type of Questions
The type of clinical question you formulate will guide you toward the most appropriate study design to answer it. Revisit your PICO question and consider its focus.
Therapy Question – Determining the effectiveness of treatment or intervention on a patient.
- Exp: Is a mediteranean diet an effective treatment to lower LDL cholesterol?
- Appropriate Study: Systematic Review, Randomized Control Trial (RCT)
Prognosis Question – Estimating a patient's likely course over time due to other factors besides interventions. This type of question looks for possible complications of a specific disease/condition.
- Exp: What is the likelihood that patients with type 2 diabetes will develop cardiovascular disease?
- Appropriate Study: RCT, Cohort Study, Case Control Study
Harm/Etiology Question – Determining the effects of potentially harmful agents on important patient outcomes. This type of question looks to find out if a harmful exposure causes a disease or condition.
- Exp: Does a poor diet contribute to type 2 diabetes?
- Appropriate Study: RCT, Case-Control Study, Cohort Study
Diagnosis Question – This type of question is about determining the accuracy of a diagnostic test.
- Exp: How accurate is a home urine test compared to a blood test to detect an early pregnancy?
- Appropriate Study: Prospective, Blind comparison to gold standard, cross sectional
Prevention Question – This question looks to identify and modify risk factors to help mitigate the risk of disease and how to diagnoe early by screening.
- Exp: How effective is routine cholesterol screnin in preventing cardiovascular events in adults over 40 with no symptoms?
- Appropriate Study: Systematic Review, Randomized Control Trial (RCT)
Example
If I have a question about improving blood pressure with a low sodium diet, then I might identify my PICO question as follows:
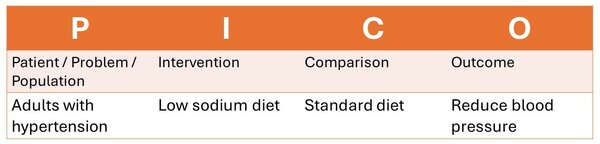
With my final question being: In adults with hypertension, does a low-sodium diet compared to a standard diet reduce blood pressure?
Because I am investigating the effectiveness of a treatment or intevention, this means this is a therapy question and therefore I should be looking for systematic reviews and randomized control trials.
Further Reading
Stillwell, S. B., Fineout-Overholt, E., Melnyk, B. M., & Williamson, K. M. (2010). Asking the Clinical Question: A Key Step in Evidence-Based Practice. The American Journal of Nursing, 110(3), 58–61. https://doi.org/10.1097/01.NAJ.0000368959.11129.79
The EBP Pyramid
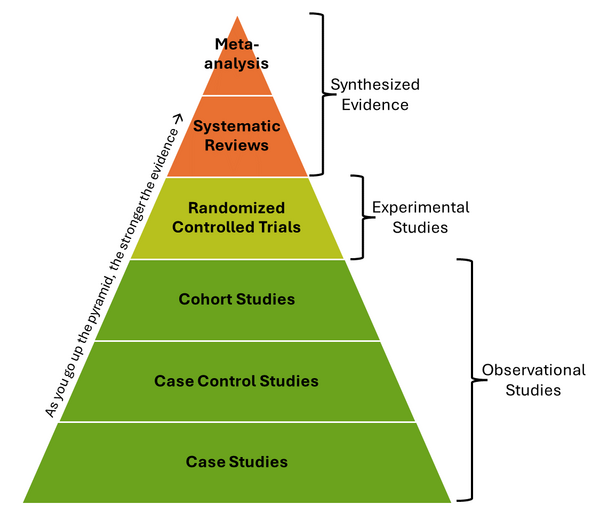
Types of Study
Meta-Analysis: A meta-analysis is a subset of a systematic review that combines data from multiple qualitative and quantitative studies to produce a single conclusion with greater statistical power.
Systematic Review: Systematic reviews address a specific clinical question by conducting a comprehensive literature search to gather all relevant studies. These studies are then reviewed, assessed for risk of bias, and their results are synthesized.
Randomized Controlled Trial (RCT): In RCT studies participants are randomly assigned to either an experimental group or a control group. This design minimizes bias and allows for a direct comparison between interventions and control conditions.
Cohort Study: Cohort studies follow large groups of individuals who have been exposed to a particular treatment or factor over time. Outcomes are compared with a similar group that has not been exposed, helping to identify associations.
Case-Control Study: In a case-control study, researchers compare individuals with a specific condition (cases) to those without the condition (controls). They retrospectively examine exposures or factors that may be associated with the outcome.
Case Studies: Case studies describe the treatment and outcomes of individual patients. They do not include control groups and lack statistical validity.
Further Reading
Grimes, D. A., & Schulz, K. F. (2002). An overview of clinical research: the lay of the land. Lancet (London, England), 359(9300), 57–61. https://doi.org/10.1016/S0140-6736(02)07283-5
Databases
CINAHL Plus
Primary index to the international literature of nursing and health.
PubMed
Provides access to citations for biomedical articles from Medline and life science journals. Citations may include links to full-text articles.
Embase
It includes full-text indexing of drug, disease and medical device data, supported by the Embase thesaurus Emtree, which facilitates precise searching so users may find the answers they need to their research questions.
PsycARTICLES
A source for full-text, peer-reviewed scholarly and scientific articles in psychology.
PsycINFO
Abstracts of scholarly journal articles, book chapters, books, and dissertations, in behavioral science and mental health. Citations and summaries date back to early 1800s; journal coverage from 1887 to present. From the American Psychological Association.
Web of Science
Web of Science, published by Thomson Reuters, is a multi-disciplinary database that provides integrated access to over 8,000 key research journals indexed in: Science Citation Index Expanded, Social Science Citation Index, and Arts and Humanities Citation Index.
SCOPUS
Covering the life, physical, health, and social sciences, Scopus is a large abstract and citation database of research literature and web sources.
Point of Care Tools
DynaMed
DynaMed is the next-generation clinical reference tool physicians can rely on for fast, easy access to point-of-care decision support. Written by a team of specialized physicians and researchers, content is updated several times daily to include information on the latest evidence-based research, providing practice-changing answers to clinical questions with optimized speed.
UpToDate
UpToDate is an evidence-based, physician-authored clinical decision support resource combining broad coverage of adult primary care, subspecialty internal medicine, ob/gyn, general surgery and pediatrics. Contains over 77,000 pages of original, peer-reviewed text that are written, reviewed and continuously updated by over 5,100 physicians to ensure that the content remains current. The expert faculty synthesizes the most recent medical information in order to provide users with specific, practical, evidence-based recommendations for diagnosis and treatment that have been proven to improve patient care and quality.
Systematic Review Databases
Cochrane Library
Collection of health databases providing independent evidence to inform clinical treatment decisions and other health related decisions.
Joanna Briggs Institute Best Practice
The Joanna Briggs Institute EBP Database allows you to search simultaneously, a wide range of summarized and appraised evidence, to inform your practice. This comprehensive range of resources includes over 3,000 records across seven publication types: Evidence Based Recommended Practices, Evidence Summaries, Best Practice Information Sheets, Systematic Reviews, Consumer Information Sheets, Systematic Review Protocols, and Technical Reports.
Appraisal Tools

CASP Critical Appraisal Checklist: The CASP website offers free downloadable worksheets you can use to critically appraise various types of articles.
Duke's Critical Appraisal Worksheets: These worksheets from the Duke University Medical Center Library will help you determine which articles are good to include in your review.
JBI Critical Appraisal Tools: JBI’s critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers.
Cochrane Risk of Bias Tool (RoB 2): This tool is used to asses the risk of bias in randomized trials.
Newcastle-Ottawa Scale (NOS): This tool is used to asses the risk of bias in non-randomized studies. On the website they have a manual and a scale to follow.
QUADAS-2: This is a quality assessment tool for diagnostic accuracy studies and recommended for Systematic Reviews.
AMSTAR-2: Standing for A MeaSurement Tool to Assess systematic Reviews, AMSTAR aims to to help create high-quality reviews by focusing on their methodological quality.