Accessibility Options:
Welcome
for Systematic Reviews!

Subject Specialist
-

Saily Marrero
Nursing & Health Studies, Biology, and Psychology Librarian
305-284-2040
Please provide a brief description of the topics or issues you would like to discuss during our consultation.
Ask-a-Librarian
What are Systematic Reviews?
What are Systematic Reviews?
Systematic Reviews are a type of evidence synthesis review primarily used in healthcare that delivers a "meticulous summary of all available primary researach in response to a research question." The idea is to capture all existing research on a topic to arrive at a consensus.
The key features of a systematic review are:
- clear aims with predetermined parameters for eligibility
- transparent and reproducible methods
- rigorous search designed to find all studies
- an assesment of the validity of the selected studies
- a systematic presentation and synthesis of the selected studies
Clarke, J. (2011). What is a systematic review? Evidence Based Nursing, 14(3), 64. https://doi.org/10.1136/ebn.2011.0049
Other Research Guides
Below you will find links to other library guides about systematic and other evidence synthesis reviews.
- Systematic Review Guide [University of Texas]
- Scoping Review Guide [University of Texas]
- Systematic Reviews, Scoping Reviews, and other Knowledge Synthesis [McGill University]
- Systematic vs. Scoping vs. Integrative Review [Duquesne University]
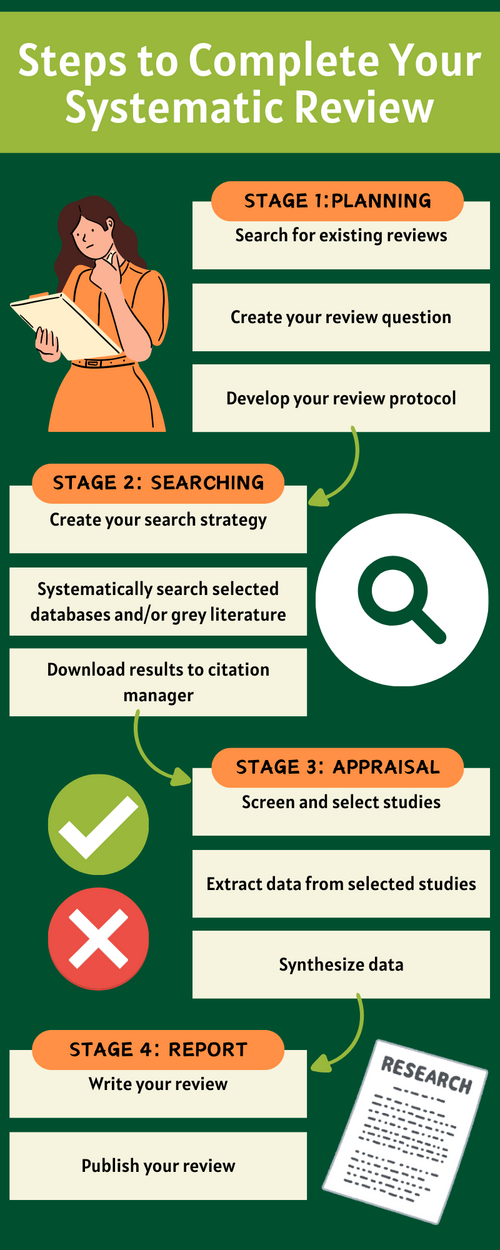
Steps to Complete Your Systematic Review
Cochrane Interactive Learning
Cochrane Interactive Learning is a an online course offered to all University of Miami Libraries users. Using the Cochrane methodology, the course is perfect for new and experienced review authors alike. It's a great introduction to systematic review for new authors while also offering more insight to the process for experienced authors.
Cochrane ON Campus Registration
ON CAMPUS REGISTRATION
1. Go to Cochrane Interactive Learning
2. Click New Users & Subscribers
3. Use the Please Log In or Create an Account button to create a Cochrane account or connect an exising accountto the University's subscription
Cochrane OFF Campus Registration
To register for a Cochrane Account Off-Campus you must register with a UM email address
- @med.miami.edu
- @rsmas.miami.edu
- @miami.edu
1. Go to https://training.cochrane.org/interactivelearning
2. Click New Users and Subscribers to register
3. On the course registration and subscription options page, the pricing information is for information only. You will not be charged. Scroll down to Institutional Access and click Please Log in or Create an Account.
4. If you already have a Cochrane Account, log in. Go to step 8
5. If you do not have a Cochrane Account, please click Sign Up Now on the account login page and complete the sign-up form.
Please note that you should register for your Cochrane Account using the UM email address validated for access to Cochrane Interactive Learning.
6. Look out for an email titled 'Cochrane Account: action required' which contains a link to activate your account. Please check your junk or spam folder in case this is misdirected.
7. When you have activated your account, you will return to the Cochrane Interactive Learning registration page.
8. You will see a notification that your email address has been validated and your registration enabled by your organization. No payment is required.
For instructions with images see this page.
Has My Review Already Been Done?
It's important to know if your Systematic Review (SR) has already been done to avoid duplication. You can search protocols of SRs on the following databases:
Apart from checking these protocol databases, search UM Library databases like PubMed, CINAHL, or PsycINFO.
Stage 1 : Planning
Develop a Research Question
Think of what research topic you are interested in and create an answerable question. Your research question should be clear and focused, but not too specific or too broad.
University of Tasmania Systematic Review Guide: This guide has various different frameworks you can use to create and develop your research question.
University of Texas Systematic Review Guide: a guide on how to to formulate and use frameworks for your research question.
Systematic Reviews of Complex Interventions: Framing the Review Question: an article on the best practice to creating a research question for complex interventions
Develop Your Protocol
Use the appropriate guideline/checklist to create and report your protocol:
- Hardin Library Protocol Worksheet: The University of Ioward Libraries created a protocol worksheet based on the PRISMA-P. This will help you develop your methodology.
- JBI Scoping Review Protocol: This section of the JBI handbook will help you develop your protocol for a scoping review
- Methodological Expectations of Cochrane Intervention Reviews (MECIR): Items C1-C23 will help you develop a protocol for your review. Overall, MECIR is a step-by-step guide on how to conduct a systematic review
- PRISMA-P: This is a checklist to report your systematic review protocol
- PRISMA-ScR: Under this PRISMA extension you will find the guidelines for reporting a scoping review protocol
Register Your Protocol
Any researcher can register a protocol. If a publication or agency does not require one, submitting one is optional, but writing one as part of the process is fundamental. It helps plan and specify how you will navigate the systematic review process and stay on course:
You can submit your protocol in any of the following databases:
- Systematic Reviews Protocols and Protocol Registries: The National Institutes of Health (NIH) has a dedicated page specifically for Systematic Reviews and further information about protocols
- PROSPERO: PROSPERO accepts registrations for systematic reviews, rapid reviews and umbrella reviews
- Open Science Framework: The OSF is used to register scoping reviews.
Guidelines for Methods
The following guidelines will walk you through each step of your evidence synthesis review project:
Joanna Briggs Institution Manual for Evidence Synthesis: This manual guides authors who want to conduct systematic and scoping reviews using the JBI methodologies.
Cochrane Handbook for Systematic Reviews: This handbook has detailed information about conducting systematic reviews as recommended by Cochrane.
Methodological Expectations of Cochrane Intervention Reviews (MECIR): MECIR provides a step by step checklist of standards with links to addtional information for conducting a systematic review or meta-analysis
Scoping Reviews: What They Are and How You Can Do Them: A collection of videos that define scoping reviews with examples, and the steps to conducting a review. You can also access the slides from presentation here.
Joanna Briggs Institution Scoping Reviews: The JBI Manual for Evidence Synthesis, section 10 is dedicated to developing and conducting scoping reviews.
Citation Managers
UM Libraries subscribes to sveral citation managers, for more information look at our Citation Research Guide.
Search Strategy Guide
To create your search strategy follow the steps in the Search Strategy Guide.
Stage 2 : Searching

Tracking Searches & Results
Overview of PRIMARY Excel Workbook for SR: Created by the University of Texas, this is a PDF guide on how to track your search data using Excel.
Saving Search Strategies with PRIMARY Excel Workbook for SR: Created by the University of Texas, this is PDF guide will help you keep track of your search strategy using Excel.
Hirsh Health Sciences Library: Use the Search Term Chart to organize, save, and track the terms used in your search strategy.
Biomedical Databases
CINAHL Plus
Primary index to the international literature of nursing and health.
Embase
Embase is a comprehensive biomedical literature database that provides over 30 million abstracts and indices from published, peer-reviewed literature, in-press publications and conferences covering the most important international biomedical literature from 1947 to the present day.
PubMed - US National Library of Medicine, NIH
Provides access to citations for biomedical articles from Medline and life science journals. Citations may include links to full-text articles.
Comprehensive index to the international literature of biomedicine including clinical practice, psychosocial issues, health care administration, medical research, nursing, and more.
Web of Science
Web of Science, published by Thomson Reuters, is a multi-disciplinary database that provides integrated access to over 8,000 key research journals indexed in: Science Citation Index Expanded, Social Science Citation Index, and Arts and Humanities Citation Index.
LILACS
LILACS database is maintained and updated by educational, research and, health institutions, from government and private sector.
African Index Medicus
In order to give access to health information published in or related to Africa and to encourage local publishing, the World Health Organization, in collaboration with the Association for Health Information and Libraries in Africa AHILA, has produced an international index to African health literature and information sources.
Psychology Databases
PsycARTICLES
A source for full-text, peer-reviewed scholarly and scientific articles in psychology.
PsycINFO
Abstracts of scholarly journal articles, book chapters, books, and dissertations, in behavioral science and mental health. Citations and summaries date back to early 1800s; journal coverage from 1887 to present. From the American Psychological Association.
ERIC (Education Resources Information Center)
ERIC is an internet-based digital library of education research and information sponsored by the Institute of Education Sciences (IES) of the U.S. Department of Education. ERIC provides access to bibliographic records of journal and non-journal literature from 1966 to the present. The ERIC online system provides the public with a centralized site for searching the ERIC bibliographic records (citations, abstracts, and other pertinent data) of more than 1.6 million items indexed since 1966.
Systematic Review Databases
Cochrane Library
Collection of health databases providing independent evidence to inform clinical treatment decisions and other health related decisions.
Joanna Briggs Institute Best Practice
The Joanna Briggs Institute EBP Database allows you to search simultaneously, a wide range of summarized and appraised evidence, to inform your practice. This comprehensive range of resources includes over 3,000 records across seven publication types: Evidence Based Recommended Practices, Evidence Summaries, Best Practice Information Sheets, Systematic Reviews, Consumer Information Sheets, Systematic Review Protocols, and Technical Reports.
ClinicalTrials.gov
Database of clinical trials sponsored by the National Institutes of Health, other Federal agencies and the pharmaceutical industry. Provides status of trials.
Agency for Healthcare Research and Quality
The Agency for Healthcare Research and Quality (AHRQ) publishes evidence-based information on health care outcomes, quality, cost, use, and access.
Science Databases
SCOPUS
Covering the life, physical, health, and social sciences, Scopus is a large abstract and citation database of research literature and web sources.
Web of Science
Web of Science, published by Thomson Reuters, is a multi-disciplinary database that provides integrated access to over 8,000 key research journals indexed in: Science Citation Index Expanded, Social Science Citation Index, and Arts and Humanities Citation Index.
ScienceDirect
Science Direct is a full text database offering journals and books from the leading scholarly publisher Elsevier. It has particular strengths in medicine and mathematics, but its nine million articles cover a wide range of scientific topics. The collection stretches back to 1823 and includes prominent publications including The Lancet, Cell and Tetrahedron.
SciELO
SciELO is a free database containing the full text of selected scientific and social sciences journal articles from Brazil, Chile, Cuba, Spain and Venezuela. Articles are available in one of three languages: English, Portuguese, or Spanish. Although it has a few journals in the social sciences, it is primarily a source for the biological, environmental, and physical sciences.
What is Grey Literature?

Grey literature is information not commercially published.
"Grey literature stands for manifold document types produced on all levels of government, academics, business and industry in print and electronic formats that are protected by intellectual property rights, of sufficient quality to be collected and preserved by library holdings or institutional repositories, but not controlled by commercial publishers i.e., where publishing is not the primary activity of the producing body."
From the Second International Conference for Grey Literature in 2010
What are some examples of grey literature?
Grey literature includes but is not limited to:
- Reports from government agencies, organizations, societies, etc. (exp: CDC)
- Primary Resources: Manuscripts, Notes, Letters, Diaries, etc.
- Professional and Special Interest Groups
- Health Institutes
- Research Centers
- Conference proceedings
- Listservs
- Blogs
- Emails
- Newsletters
- Theses & Dissertations
For a full list of Grey Literature Documents visit GreyNet.org
Grey Literature Resources
CADTH (Canadian Agency for Drugs and Technologies in Health): Canada's Drug Agency is a health organization that provides independent evidence and advice for drug, health technology, and health system decisions.
CADTH's Grey Matters: This is a free resource for finding health-related grey literature that is not published commercially.
U.S. Department of Health & Human Services (HHS.gov): The HHS has information on different programs, services, laws, and policies
Centers for Disease Control and Prevention (CDC): Has information, data, and tracks different health topics in the United States.
ClinicalTrials.gov: You can search for different clinical studies currently being done around the world.
Health & Medical Resources (Florida Atlantic University Libraries Research Guide): Has a list of websites and resources about different health topics.
Healthy People: The HHS Healthy People initiative has data illustrating their goals and evidence based resources.
National Center for Health Statistics (NCHS): The U.S.'s principal health statistics agency providing insights into the health of people and how it has changed over time.
PEDro: The physiotherapy evidence database includes trials, review, and guidelines evaluating physiotherapy interventions.
Sigma Respository: Encourages nurses at any point of their career to submit their work with them. They are the only repository dedicated to sharing work created by nurses worldwide.
Trip Medical Database for Evidence Based Practice: A clinical search engine designed to allow users to easily find quality evidence to support their clnical decisions and/or care.
World Health Organization (WHO): The WHO provides information, resources, and data on different health topics affecting people worldwide.
WHO International Clinical Trials Registry Platform: A place where researchers can submit and ensure a complete view of research is accessible to all those involved in health care decision making.
AID/HIV Resources
AIDS.gov: This website provides relevant information on a variety of HIV related topics like statistics, policies, programs, and resources across the U.S. government
HIV Source: This website is a portal for HIV related information found within and outside of the U.S. federal governement as well as other countries.
CDC HIV: The CDC has their own pages dedicated to information about HIV/AIDS
CDC's NCHHSTP: The CDC's National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention (NCHHSTP) has the AtlasPlus app where you can view and track diseases by year, geography, and demographics.
COVID-19 Resources
CDC COVID Data Tracker: The CDC has been tracking COVID-19 infections in the United States and offers more information on COVID-19 related topics.
COVID END: This was a time-limited network that brought together the world's leading research groups to cover the needs of the pandemic response for public-health and clinical settings.
COVID 19 Research Guide: The Calder Library at the Miller School of Medicine has an entire guide dedicated to COVID-19 and the resources available.
Health Disparities
MedlinePlus - Health Disparities: This website has an introductory information on health disparities accompanied by research articles, websites, and statistics on the topic.
U.S. Department of Health - Office of Minority Health: This government run website
EthnoMED: This website provides information about cultural beliefs, medical issues, and other immigrant health related topics.
Covidence

Covidence is a web-based software designed to facilitate systematic review projects. With this software, systematic review teams can screen citations, full text review, risk of bias, and etc for a systematic review. Covidence is available for all UM students, faculty, and staff.
TUTORIALS & GUIDES
Stage 3 : Appraisal

Join University of Miami Library's Covidence Institutional License
You can create your personal sign in information with Covidence before or after joining the institutional subscription. To request access to the institutional account in Covidence, you must use your currnt UM email address (i.e. @miami.edu)
- Go to the Covidence sign-up page
- Enter your information using your UM email and click “Request Invitation” link
- Accept the invitation in your email
- Log in to your existing Covidence account or sign up for a new account
- If you have already joined the University of Miami Libraries’ Covidence account, then you can log into Covidence and with your email and password and proceed to use Covidence
Create a Review Using UML's Unlimited License
- Once logged in click on "Start a new review". You have the option to use your personal account or UM account to create a review.
- Reviews created under the UM account can be seen by administrators of the University of Miami Libraries Covidence account. The reviews under your personal account will only be seen by you.
- Once you have have created or accepted an invitation to another UM account review, the titles will appear in separate sections of your homepage as seen below

Appraisal Tools

Critical Appraisal Worksheets: These worksheets from the Duke University Medical Center Library will help you determine which articles are good to include in your review.
JBI Critical Appraisal Tools: JBI’s critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers.
Cochrane Risk of Bias Tool (RoB 2): This tool is used to asses the risk of bias in randomized trials.
Newcastle-Ottawa Scale (NOS): This tool is used to asses the risk of bias in non-randomized studies. On the website they have a manual and a scale to follow.
QUADAS-2: This is a quality assessment tool for diagnostic accuracy studies and recommended for Systematic Reviews.
AMSTAR-2: Standing for A MeaSurement Tool to Assess systematic Reviews, AMSTAR aims to to help create high-quality reviews by focusing on their methodological quality.
Reporting Guidelines
PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) is a checklist used for reporting systematic reviews and meta-analysis. It has its own website where you can access the reporting guidelines as well as the extensions for protocols, searching, scoping reviews, among others.
PRISMA-ScR: This PRISMA extension is a checklist used to report scoping reviews.
PRISMA-Search: This is a PRISMA extension used to report your search strategy.
Stage 4 : Report

Writing Center
If you need assistance with writing book a consultation with the Writing Center they will help you at any point of the writing process. They are available for in-person visits and virtually.
